.jpeg)
Your doctor may suggest participating in a clinical trial to explore new or improved treatments. These trials aim to find out whether new approaches work better than current ones.
If you're considering joining a trial, it can be helpful to talk with your specialist, GP, or even seek a second opinion. Your healthcare team can help you understand what’s involved and whether a trial is right for you.
Taking part in a clinical trial is completely voluntary. If you choose to participate, you can change your mind and withdraw at any time.
Clinical trials are a vital part of cancer research. They help doctors and scientists learn which treatments work best and are safest - not just for medical treatments, but also for psychological and supportive care. Over time, clinical trials have led to better treatments and improved outcomes for people diagnosed with cancer.
One common type of trial is a randomised controlled trial. In this kind of study, participants are randomly assigned to receive either the current best treatment or a new treatment. This helps researchers find out if the new approach works better.
Clinical trials can test many different types of interventions, including:
Clinical trials are often completed in phases, starting from a small number of participants and increasing the number during each phase.
There are four main phases of clinical trials:
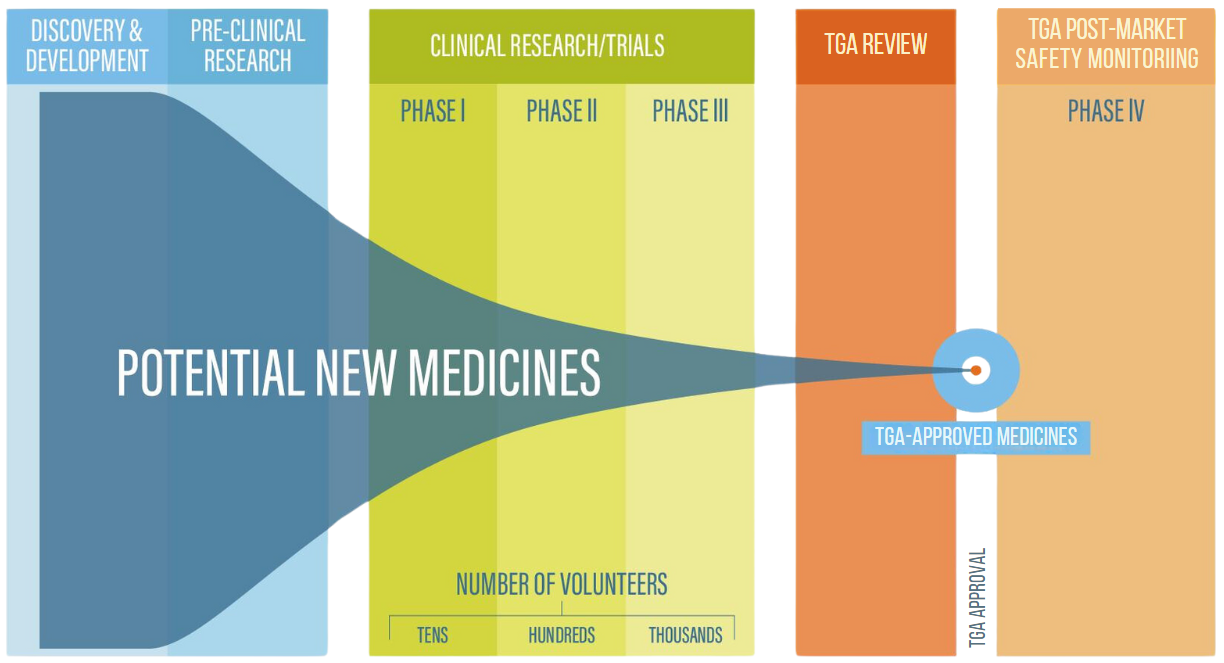
Australian Clinical Trials Government resource explaining how clinical trials work.
Clinical Trials - Cancer Council
Information about clinical trials including those specific to cancer.
You can be referred to a clinical trial by your treating doctor. They can check which trials are currently recruiting and whether you might be eligible. If a suitable trial is available, your doctor can refer you to the hospital where the trial is being run.
If you prefer to search online, there are websites that list clinical trials currently recruiting in Australia. These resources can be helpful, but they may be difficult to navigate - especially for people with a suspected or confirmed diagnosis of CUP. Many trials have strict eligibility criteria, such as requiring a specific primary cancer site or genetic mutation.
We strongly recommend discussing any trial options with your treatment team, who can help guide you through the process.
Here are some trusted websites where you can search for clinical trials:
Australian New Zealand Clinical Trials Registry | ANZCTR
Official registry listing all clinical trials in Australia and New Zealand.
Find cancer-related clinical trials in Australia. Easy to search by cancer type or treatment.
Australian Registry of Cancer Trials
Clear information about cancer trials, developed by Cancer Australia.
Government site with general info about clinical trials and how to get involved.
The CUPiD study is exploring how advanced genomic testing can help identify the possible origin of cancers that do not have a known primary site. This testing is done on a biopsy sample - usually one that has already been taken. In some cases, a new biopsy may be needed if the original sample is not suitable. Finding a potential primary cancer site can help your doctor tailor treatment more specifically to your needs. Please note: this study is for testing purposes only and does not provide treatment.
You may be eligible to participate in the CUPiD study if you:
✅ Have a suspected or confirmed diagnosis of CUP
✅ Are not currently receiving treatment through this study - it is for testing only
✅ Have a biopsy sample available (usually previously taken).
In some cases, a new biopsy may be needed if the original sample is not suitable.
You may not be eligible if:
❌ Your cancer has spread from a known primary site.
❌ You have had previous test results that clearly point to a specific cancer type or origin.
This study is currently recruiting at the following hospitals:
· Royal Adelaide Hospital (SA)
· Flinders Medical Centre (SA)
· Queen Elizabeth Hospital (SA)
· Lyell McEwin Hospital (SA)
· Mount Gambier and Districts Health Service (SA)
· Royal Darwin Hospital (NT)
If you're considering joining a clinical trial, please speak with your treating doctor. They can help determine whether a trial is appropriate for you and whether you meet the eligibility criteria.
The FAPI-CUP study is testing a specialised type of PET scan, called the 68Ga-FAPI-PET-CT scan, to help find out where a cancer may have started. This scan is different from the standard FDG-PET scan and may be more effective at detecting certain tumour types - some of which are linked to CUP.
You may be eligible if you:
✅ Have a confirmed diagnosis of CUP
✅ Have completed your diagnostic workup (standard tests to try to find the cancer’s origin)
✅ Are well enough to undergo a PET scan (your doctor will confirm this)
✅ Are 18 years or older
You may not be eligible if you have:
❌ Already started your line of cancer treatment
❌ Had major surgery in the last 6 weeks
❌ A medical condition that could affect the scan or your safety
❌ A cancer diagnosis in the last 3 years (some exceptions apply)
❌ An allergic reaction to PET scan tracers (such as 18F or 68Ga)
This study is currently recruiting at:
FAPI-CUP Study | Clinical Trials Australia
If you're considering joining a clinical trial, please speak with your treating doctor. They can help determine whether a trial is appropriate for you and whether you meet the eligibility criteria.
The CaSP program offers Comprehensive Genomic Profiling (genetic testing) for people in Australia who have been diagnosed with an advanced, incurable, or poor prognosis cancer.
This testing uses a previous tumour sample (or sometimes a blood sample) to look for genetic changes that may help your doctor:
You may be eligible if:
✅ You have a confirmed cancer diagnosis
✅ Your treating doctor refers you and you provide consent
✅ A tumour tissue sample is available (or you are willing to provide a new one if needed)
Once your sample and information are reviewed, your results will be available in 8–10 weeks.
You may not be eligible if:
❌ You have a medical condition that could affect the accuracy of the genomic testing
❌ You were diagnosed with another cancer within the past 2 years, unless it was treated and you’ve been cancer-free for at least 6 months
CaSP is a nationwide program available across Australia.Testing is coordinated through NATA-accredited pathology laboratories, and referrals are made by your treating oncologist.
You can access CASP through many public and private cancer centres that are part of OMICO’s national network.
Cancer Screening Program (CaSP) | Omico
If you're considering joining a clinical trial, please speak with your treating doctor. They can help determine whether a trial is appropriate for you and whether you meet the eligibility criteria.
Here are the links to other information pages to learn more about different aspects of treatment. You may also use the quick links on the right side of the page to navigate.

Most people are diagnosed with cancer of unknown primary (CUP) after they have symptoms or become unwell. Some people may be diagnosed during tests for another health condition. When cancer is suspected, you might be referred for tests or to a specialist.

The treatment you have depends on a number of things, including where the cancer is and your general health. A team of doctors and other professionals discuss the best treatment and care for you. The main treatment for Cancer of Unknown Primary is cancer drugs, most commonly chemotherapy. You may also have radiotherapy to help to control your symptoms and hormone therapy.




